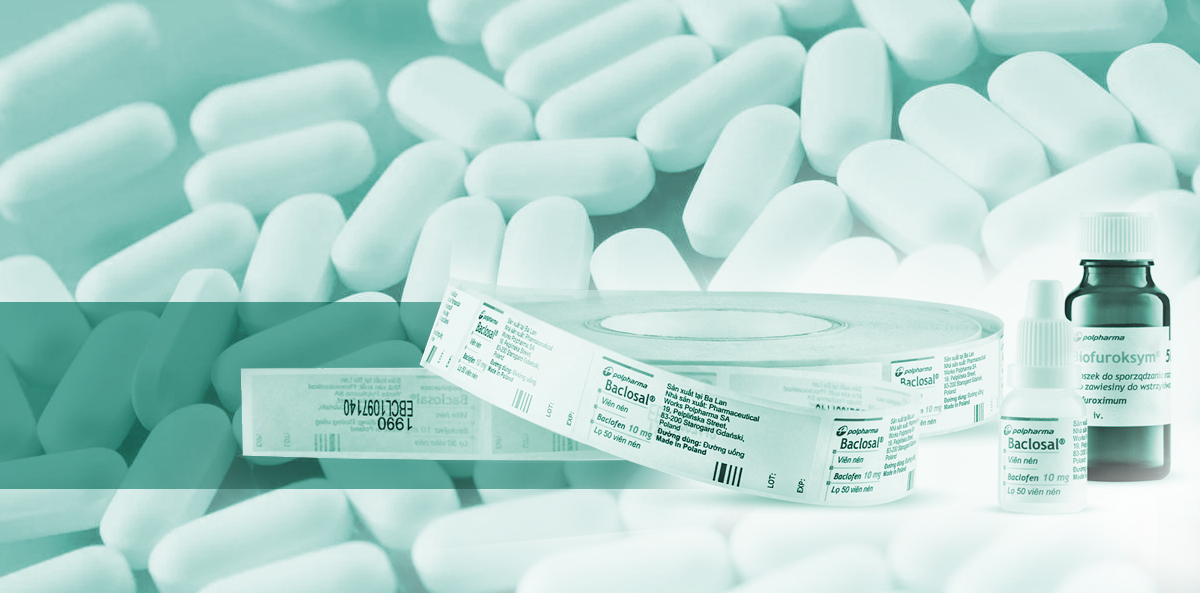
Protective labels for pharmaceutical packaging
Pharmaceutical manufacturers are supported by Etisoft in the serialisation process
Serialization in the pharmaceutical industry is about to take place. From 9 February 2019, pharmaceutical manufacturers will have to ensure that their products are authentic and that their reliability can be confirmed at every stage of the supply chain.
The EU directive 2011/62/EU, known as the Falsified Medicinal Product Directive, made it mandatory to place safety features (e.g. in the form of safety labels) on the packaging of medicines against their falsification. Most importantly, these
As a result, the new requirements are intended to allow inspection units and consumers, in particular, to distinguish an authentic medicine from a counterfeit one, and to reveal any attempts to open the outer packaging of the medicine.
Three types of tamper-evident labels – which one to choose?
In the serialization process, pharmaceutical manufacturers are supported by Etisoft with several types of tamper-evident labels. If you try to detach the label from the surface of the packaging, you can see if it has been tampered with. Sealing materials also leave a visible trace on the packaging, protecting it against unauthorized opening.
Our anti-counterfeiting product portfolio includes a wide range of solutions, including:
- strong adhesive labels with high initial adhesion and high adhesion on a wide range of substrates, including lacquered surfaces. This protection destroys the top layer of the packaging, making it easy to determine whether its integrity has been breached.
- crushing labels – with a durable adhesive for high initial adhesion on a wide range of substrates, including lacquered surfaces. The product has been designed to detect any manipulation attempts. The transparent film is very brittle. As a result, in combination with a strong adhesive, it is extremely easily damaged and difficult to remove from the surface of the packaging.
- VOID type labels – leaving a mark on the packaging in the form of the inscription “VOID” when trying to remove them. The labels have been designed in such a way that after being peeled off the surface and re-applied, even in the same place, it is noticeable that they have been manipulated.
How to protect pharmaceutical packaging effectively with labels?
All of our tamper-evident labels are primarily
Our team of technologists assists manufacturers in choosing the right solution to ensure its functionality. We also help in the implementation of the solution on packaging lines. Thus, Etisoft supports the safety of medicine users and the integrity of the manufacturer’s brand on the pharmaceutical market.
The serialization process will be introduced in most European countries and imposes medicine verification obligations on wholesale distributors, pharmacies and persons authorized to supply medicinal products to the market.
The serialization process will be introduced in most European countries and imposes medicine verification obligations on wholesale distributors, pharmacies and persons authorized to supply medicinal products to the market.
(176)